
Feb . 19, 2024 12:07 Back to list
Tinplate-WHAT IS TINPLATE?
Tinplate Testing
WHAT IS TINPLATE?
The tests which may be applied to tinplate can broadly be classified into two groups, objective, or quality tests and subjective, or performance tests. Many of these tests, especially the former, are routinely carried out during manufacture, as part of a quality control programme. Other, more sophisticated tests are used for research purposes or to investigate problems which may have arisen in service. The validity of any testing procedure is based on the assumption that the samples selected are representative of the bulk of the material to be tested. Sampling procedures for selecting sheets for mechanical property and coating thickness tests are outlined in the international and national standards.
- MECHANICAL PROPERTY TESTS
A number of different mechanical properties may be determined for tinplate. No single mechanical test can measure all the factors which may determine its performance, nor is it possible to apply any single test satisfactorily to the range of tinplate products that is available on the market. The term “temper” which is used to classify tinplate products, summarises a combination of interrelated properties. The performance of tinplate in processing and use is significantly determined by individual mechanical properties, which will vary on the steel type, methods of casting, rolling, annealing, temper rolling employed.
The test considered to provide the best overall guide to mechanical properties is the tensile test. This test is often preferred as the referee method. For more routine testing, the Rockwell hardness testing method is used for single-reduced material and the Springback test is used for double-reduced tinplate.
Other, more specific tests can be used to set additional demands on the forming properties of tinplate.
- THE TENSILE TEST
The conventional tensile test provides the more accurate measure for the tensile properties of tinplate, but is relatively slow and requires very careful skilled preparation of the test specimens
The test consists of straining a specially prepared test piece, having parallel sides, by tensile stress, generally to fracture. As the tinplate is strained, it elongates, initially in proportion to the load applied. At a certain point (the limit of elasticity), the elongation proceeds more rapidly. The proof stress (Rp) is defined as the stress at which a non-proportional elongation equal to a given percentage of the original gauge length occurs. For tinplate testing the value customarily measured is 0.2% (Rp0.2). The tensile test is performed in the normal way, using wedge grips, but due to the light gauges commonly used for doublereduced tinplate, certain special precautions should be observed.
It is advisable to finish the preparation of the edges carefully with fine emery paper, to remove any burrs.
The gauge length (effective length with parallel edges) used is 50 mm ± 0.5 mm and the width 12.5 mm ± 1 mm.
The rate of straining is usually 1 mm/min.
The grips employed should be capable of securing the sample such that no skew occurs during straining, to ensure that the applied stress is aligned centrally along the major axis of the test specimen.
The value recorded is the proof stress (Rp0.2).
- HARDNESS
The Rockwell Superficial Hardness Test is used to assess single-reduced tinplate temper grades; the hardness ranges specified for the various temper grades are given in C hapter Ill. This test is specified in international standards, normally on de-tinned specimens.
The Rockwell hardness tester is a direct reading instrument which measures the incremental depth of penetration of a ball indentor forced into the metal by a primary and a secondary load. In the Superficial Tester, as used for tinplate, the minor load is 3 kg and the major load a total of 30 kg or 15 kg. The loads are applied via a system of levers and at a controlled rate. The hardness value is read directly on a calibrated dial.
To carry out the test, place the specimen on the diamond centre spot of the anvil, avoiding testing near the edges of the specimen because of a possible cantilever effect. Bring the specimen into contact with the ball indentor by turning the hand wheel until the indicator on the dial shows that the minor load is applied. Then turn the adjustable rim of the dial until the pointer reads zero, and apply the major load by operating the handle. The rate of loading is controlled by a dash-pot incorporated in the machine. As soon as the loading is complete, remove the major load by pulling the handle forward and read the Rockwell hardness number on the appropriate scale.
Due to the light gauge of tinplate sheet, the Rockwell 30T Hardness test is sensitive to the “anvil” effect in which the measured apparent hardness is affected by the thickness of the test specimen; for very thin sheet (e.g. < 0.20 mm.) a 15 kg major load (15T Scale) may be used as described in the relevant standards.
Samples of continuously annealed material used for Rockwell hardness testing must be adequately aged. Artificial ageing will normally be necessary where the material has not been pre-stoved through a lacquering or printing process. Artificial ageing can be achieved by heating the specimen to 200OC for 20 minutes.
Other hardness tests used for sheet metal (e.g. Vickers) are not advised for tinplate testing since it is not possible to convert values accurately to Rockwell values.
- THE SPRINGBACK TEST
The Springback test was devised to enable the proof stress of double reduced tinplate to be determined conveniently. It is frequently used as a routine test and is also cited in the appropriate standards.
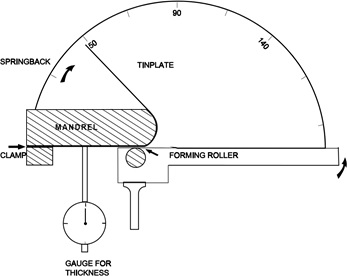
Principle of operation of a springback tester
To perform the test, a tinplate specimen 150 mm x 25 mm wide, of known thickness is clamped at one end and the free end is bent through 180 deg. around a 25 mm diameter mandrel with the aid of a forming roller pivoted so as to follow the mandrel (Figure 31). The free end of the specimen is then released and the springback angle read directly on the calibrated scale.
The amount of the springback angle and the thickness of the specimen are then used to obtain the “springback index” in terms of the proof stress. Suitable conversion formulae are published for this purpose.
- FORMABILITY TESTS
In addition to the tests which are stipulated in specifications, tinplate manufacturers and users customarily carry out certain other tests on the steel base to assess the suitability of the product for a given application. Among these tests are the following.
- FLUTING TEST
In the manufacture of three-piece cans the rectangular body blank is formed into a cylindrical body. If the correct tinplate grade is not used the resultant cylinder will not be of smooth appearance, but the body wall will exhibit “flutes” or flat irregularities in its circumference. To check this the so-called fluting test is used.
In this test the specimen is simply formed into a cylinder of about 50mm diameter by a standard three-roll forming machine. After forming, the cylinder is examined visually for evidence of “flats” or irregularities in the circumference of the cylinder. This type of test is normally applied only to those grades of tinplate used for circular bodies where fluting is undesirable.
- BEND TESTS
The simplest form of bend test used for sheet metal is the single bend test in which a specimen is doubled on itself and hammered flat, to induce cracking. This type of test is not sufficiently sensitive for tinplate, all grades of which bend and seam readily. However, since in fabricating, for example a can body, side seaming and end flanging involve bending the sheet both parallel and transverse to the rolling direction, it may be necessary to assess the degree of directionality of its forming properties. This may be determined by an alternating bend test applied to specimens cut along the rolling direction (“strong way”) and transverse to it (“weak way”).
- THE JENKINS ALTERNATING BEND TEST
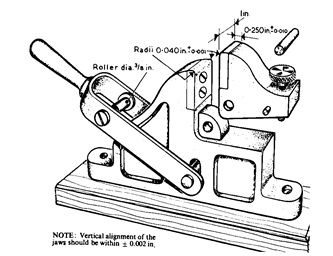
Figure General view of the Jenkins bend tester
In this test a strip of tinplate, approximately 60 mm long by 12 mm wide is clamped between accurately radiused jaws and the free end is repeatedly bent to and fro through 180° until failure occurs. In the Jenkins machine the specimen is bent by a fixed diameter roller operated by a hand lever, the end point being reached at the first sign of fracture through the specimen (Figure). The number of full 180° bends required to produce the fracture is recorded as the Jenkins Bend Value (the first half bend is ignored). It depends on the temper grade and the sheet thickness.
When carrying out an alternating bend test, two specimens should always be taken, one cut with its long edges parallel to the rolling direction and the other at right angles to this direction. The difference between the two Bend Values and the magnitude of the “weak way” value are both of significance and are particularly useful in estimating bending and flanging properties.
Several more sophisticated machines for performing alternating bend tests have been devised on the same principle.
- CUPPING TESTS
The drawability of sheet metal is commonly assessed by means of cupping tests. The principle underlying all cupping tests is that a cup is formed in the sheet specimen by means of a punch and die set mounted in a suitable press, the sheet being clamped by a pressure plate or mounting ring. The cupping value is normally taken as the depth of impression required to produce fracture, although other criteria may be used, e.g. the drop in load on the punch which occurs at the moment of fracture.
Cupping test values are not normally included in tinplate specifications, but for routine testing the test most frequently used for tinplate is the Erichsen cupping test.
Erichsen test
In the Erichsen test the test piece is a 76 mm diameter blank, held between an annular ring (blankholder) and the die; the punch is a 20 mm diameter hemisphere and it is advanced against the specimen mechanically or hydraulically to form a depression or “cup”. The end point is normally taken as the point at which the cup wall just fractures. The depth of impression, in millimetres, is the Erichsen value. It is related to the tinplate temper grade and the thickness of the material.
The Erichsen test method is defined in various national and international specifications. For tinplate the standard method may be used except that, since tinplate carries an oil film, no additional lubrication is applied to the test specimen.
- METALLOGRAPHY
The steel base of tinplate may be examined by standard optical micro-examination procedures. Features of special interest are the shape and size of the grain structure, to check the temper grade and the correctness of the annealing cycle; and the degree and distribution of non-metallic inclusions, which can affect formability.
Electron microscopy and scanning electron microscopy are widely used to study the continuity of the alloy coating on tinplate.
Electron microscopy and scanning electron microscopy are widely used to study the continuity of the alloy coating on tinplate.
The coating of tinplate
The majority of tinplate is flow-melted. The resultant coating is therefore composite, comprising a thin layer of tin over an even thinner layer composed of the alloy FeSn2 formed by reaction between the electroplated tin and the steel substrate. Each layer is metallurgically and tightly bonded to the adjacent one.
The passivation treatment customarily applied to electrolytic tinplate, is intended to convert part of the naturally occurring tin oxide film into a layer containing chromium and chromium oxides. This treatment is beneficial in improving both storage and lacquering performances. It is important to be able to characterise the passivation films.
Both the tin coating of tinplate and the surface films, may be determined by coulometric techniques. Before describing the details specific to a given determination, it is of interest to consider, in general terms, the principles of the coulometric technique.
- COULOMETRIC METHODS - GENERAL
Several of the laboratory inspection tests used for tinplate may be made by measurement of the charge passed in the performance of some particular oxidation or reduction. For this type of test, the basic equipment is always the same: a source of controlled constant current, a suitable voltage recorder and an electrolysis cell which can contain the test sample, a counter-electrode, usually of platinum or carbon, and a reference electrode, usually silver/silver chloride or saturated calomel. Modern equipment usually integrates the voltage source and measuring functions in a computer controlled unit, which also has datahandling, storage and output functions. The solutions used, the current densities and the direction of current, and the range of voltage to be measured differ from test to test, but it is possible to use the same general equipment for various tests. Therefore, a preliminary description of the basic apparatus is given now so that it need not be repeated in dealing with the several tests for which it may be used. It should be mentioned, that commercially available coulometric instruments for coating thickness measurement, are usually not easily adaptable to other measurements. However, the electrolysis cell, one of the most important parts of the apparatus, is often general purpose.
- ELECTROLYSIS TEST CELL
Diagram of simple cell for coulometric measurements on tinplate.
The components are:
The components are:
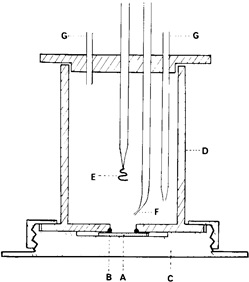
- A-test piece
- B-O-ring
- C-brass base
- D-cell body, a cylinder of transparent plastics material
- E-counter electrode of platinum wire
- F-tip of calomel reference electrode
- G-inlet and outlet tubes for inert gas. Electrical connection to the test piece is made through the brass base
The essential feature of the test cell is a holder for the test specimen which can make a watertight joint around a precisely measured area of surface. This is accomplished almost invariably by lining the edge of an aperture cut into plastics material with an O-ring or other form of gasket against which the test piece is pressed by tightening a screw thread, by a spring or by other means. A common form of holder is a cylinder made of Perspex of length about 3 cm, with a circular aperture in one end and closed at the other end by a threaded plug which presses a tinplate disc of 5 cm diameter against a gasket surrounding the aperture. Electrical connection to the test specimen is by a light spring connected to a wire passing through an insulated sleeve attached to the holder. A holder of this type is separate from the cell and must be held in place during tests, but has the advantage that electrolyte can be left in the cell between tests. However, many laboratories have preferred to use a cell in which the test specimen is exposed through an aperture at one end of the cell. This method has advantages in that the position of the test specimen is always the same, that the cell can be more easily closed for operation in oxygen-free conditions and that the test specimen does not have to be cut to a precise shape or size to fit a holder. A common type of cell used has the test specimen covering an aperture in the base of a cyclindrical cell closed at the upper end by a removable cap which carries the counter-electrode, the reference electrode and means for introducing nitrogen and test solution.
Cell bodies are made with several sizes of aperture ranging from 1 cm to 5 cm to permit tests on different areas. The use of small test areas, provided that they are precisely limited by the cell aperture, has some advantages for surveying variations across a surface and for making measurements on manufactured cans which have only small plane surfaces. The test specimen rests on a circular metal plate by which it is pressed into contact with the cell base by an interlocking collar engaging a flange on the base. A diagram of the cell is shown below. Electrical connection to the test piece is made via the metal plate.
A general circuit diagram for the operation of several forms of coulometric test with the basic equipment is shown in below figure
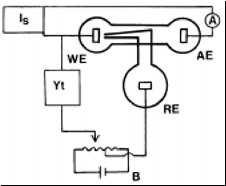
The range of currents required for testing various properties over areas of different sizes is from 25µA to 200 mA. It is possible to cover this range with one of the constant voltage/
current power sources available ready-made from laboratory suppliers if the smaller constant currents are obtained by using a constant high voltage of 100-200 V with resistance of several megohms in series with the cell and the larger currents are made to rely on direct control of the current by the source. If a general duty source is not available, the same plan can be followed using
smoothed rectified mains voltage
Circuit diagram for coulometric analysis or a dry battery as a high voltage source and batteries or other
convenient D.C . source with potential dividers to give the higher currents.
- POTENTIAL MEASUREMENT
A potential recorder with a fast response and a chart speed of at least 30 mm/ min is required and either the recorder must have the capability of showing changes up to + 1 V from base-lines ranging from -1 to 0 V or means of applying a controlled backing-off voltage must be provided. The recorder, if connected directly to the electrodes must have an input impedance of more than 1 megohm: otherwise a voltmeter of high input impedance may be used and its readings fed to a general-purpose recorder. An additional useful refinement is to obtain or make equipment which converts the signal to the recorder to the rate of change of potential with time. Such a device produces a sharpened indication of potential changes.
As mentioned, more modern potentiostats integrate the current source and measuring functions, together with the proper data-handling and analysing software.
- TIN COATING MASS DETERMINATION
The free tin and the alloy layers may be determined separately or together. For specification purposes it is the total tin (i.e. free tin plus tin-in-alloy) which is determined. However, since certain characteristics, such as solderability or corrosion resistance, may depend on the relative amounts of the two layers it is customary to determine them separately, by, for example, coulometric techniques, and to sum the results.
Most standard specifications permit any of the recognised coating mass tests to be employed as routine. Many fast and reliable methods can be used for routine measuring. Usually these methods give consistent results if used by the same laboratory under steady conditions. For arbitration purposes, wet chemistry has been proven to give the most certain results, so the Referee method (see later) must be used.
- THE COULOMETRIC METHOD
This method is widely used for the determination of both free tin and tin-in-alloy. The principle of the method is that the coating is dissolved by the action of an anodic current in hydrochloric acid and the time intervals required for dissolution of the free tin layer of the coating and of the alloy layer are indicated by changes of potential between the test piece and a reference electrode. Usually the current density on the test-piece is held constant and the charge required to remove a layer of the coating is obtained by multiplying the value of the current by the time taken for dissolution of the layer. It is possible to measure the charge directly with a wattmeter with automatic or manual separation of readings for the two layers; in this instance exact control of the current is unnecessary.
The apparatus required is that described for general coulometric methods. C urrent densities in the range 4-10 mA/cm2 are required. The reference electrode may be a silver rod or saturated calomel.
The solution used is 1.0 M hydrochloric acid. The solution may be used many times, perhaps 10-50 depending on the cell capacity. It should be changed after it has stood idle for some hours or whenever potential measurements indicate deterioration.
For research purposes, when precise measurements of potential and curve-shapes may be required, a fresh solution should be used for each determination.
- PROCEDURE
When the apparatus is first set up, and occasionally thereafter, make a dummy run with known material to establish that controls of current and the recording of the potential are satisfactorily established. The current density may range from 4-10 mA/cm2 and a value at the upper end of this range is usually suitable. The potential measured will be negative to the reference electrode and the change from the start of stripping the free tin layer to the final exposure of steel will be about 180 mV. Whether the apparatus is automatically or manually controlled, it is wise to check occasionally the calculated coating mass against reliable direct measurements for each layer of the coating. Attention should also be paid to the uniformity of removal of the coating over the tested area, and, if necessary, the position of the cathode should be adjusted.
Cut a test piece of the shape and size appropriate to the cell used and clean it cathodically in 1% sodium carbonate solution at room temperature. Place the cleaned test piece in position in the cell together with the counter-electrode and reference electrode. Introduce sufficient solution to cover the test area and the electrodes and immediately start the potential recorder and switch on the current. When coating removal is complete switch off current, remove the test piece and examine the surface for any evidence of incomplete or non-uniform stripping.
(The sequence described for placing specimen and electrodes and for introducing solutions are those suitable for the type of cell in which the specimen is held against an aperture in the cell body; the operations will be different when the specimen is held in a removable holder and the solution remains in the cell between tests.)
- RESULTS
A typical potential time curve is shown in Figure below together with a plot of the rate of change of potential against time. The ideal end points for dissolution of the free tin layer and of the alloy layer are the points of inflection before the change to the next potential step. The point of inflection is usually easily perceptible in the potential/time trace and is made sharply apparent by the differential curve which may be obtained automatically by suitable analysing software. ASTM A630-68 uses intersections of extensions of the lines of the potential steps with lines following the maximum slope during the major potential changes. However, this is frequently unsatisfactory since the final potential of the exposed steel is often not constant, but may continue to move slowly either upwards or downwards, sometimes with a pronounced cusp. The different methods for determining the points of inflection make it difficult to interpret data from different labs.
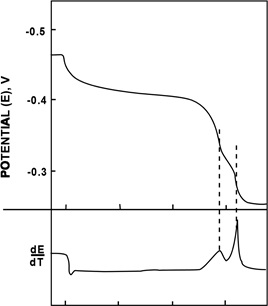
The coating thickness is calculated from the current and the time intervals on the basis that 1 C /cm2 (10 mA/cm2 for 0 60 120 180 100 sec) removes 6.15 g/ TIME (T), s m2 of free tin or 4.06 g/Potential/time curve for m2 of tin in alloyed form coulometric measurement of coating thickness (this weight of alloyed tin on tinplate with corresponding rate of change corresponds to 5.06 g/m2 of potential/time curve. tin-iron compound). A current density may be chosen and retained to provide easy direct conversion of time interval to result, but it is always possible to prepare tables or graphs to aid the conversion
- AUTOMATIC APPARATUS
Tin coating-mass coulometers are available as complete equipment. These usually react automatically to signals of potential change and convert the charge passed between changes into computer data which can be stored and analysed on the display. It is wise to check by comparison with manual methods that the response of the equipment to potential change comes at the correct place and that the conversion is accurate.
With all apparatus, automatic or manual, accuracy is much dependent on the area under test remaining well-defined; occasional checks should be made and, if necessary, gaskets and sealing rings should be changed.
X-ray fluorescence technique
In the X -ray fluorescence method, the sample of tinplate is irradiated with X -rays which penetrate the tin coating and excite fluorescent secondary radiation on the steel surface. The intensity of the emergent fluorescent beam depends on the thickness of the coating through which it passes and measurement by means of a suitable counter enables a rapid and nondestructive means of thickness determination.
There are several commercial examples of apparatus using this principle. As a non-destructive test it may be used as a laboratory tool for monitoring the coating mass on individual sheets at localised areas. The technique has also been adapted for in-line monitoring of coating thickness in electrolytic tinplate lines. In this form the apparatus customarily travels systematically across the strip width and monitors at frequent intervals the coating at selected points.
- THE REFEREE METHOD
National and international standards specify a Referee method to be used in cases of dispute. In most standards it is a volumetric method, which involves dissolution of the tin coating in hydrochloric acid. The tin in solution is reduced to the tin (II) form with pure aluminium wire, and determined by titration with standard potassium iodate-iodide solution under an atmosphere of carbon dioxide. The effective range of the method is from 2.5 g/m2 up to 50 g/m2, the reproducibility being ± 0.1 g/m2. Other (proposed) standards specify the previously described coulometric method as the referee method. 1 C /cm2 (10 mA/cm2 for 0 60 120 180 100 sec) removes 6.15 g/ TIME (T), s m2 of free tin or 4.06 g/Potential/time curve for m2 of tin in alloyed form coulometric measurement of coating thickness (this weight of alloyed tin on tinplate with corresponding rate of change corresponds to 5.06 g/m2 of potential/time curve. tin-iron compound). A current density may be chosen and retained to provide easy direct conversion of time interval to result, but it is always possible to prepare tables or graphs to aid the conversion
The detailed procedure is fully described in various national and international standards to which reference should be made. A summary of the volumetric method is as follows. 1 C /cm2 (10 mA/cm2 for 0 60 120 180 100 sec) removes 6.15 g/ TIME (T), s m2 of free tin or 4.06 g/Potential/time curve for m2 of tin in alloyed form coulometric measurement of coating thickness (this weight of alloyed tin on tinplate with corresponding rate of change corresponds to 5.06 g/m2 of potential/time curve. tin-iron compound). A current density may be chosen and retained to provide easy direct conversion of time interval to result, but it is always possible to prepare tables or graphs to aid the conversion
From each of the selected sheets, accurately punch three specimens each of an area not less than 25 cm2, one from the centre of the sheet and the other two from diagonally opposed corners. Degrease these specimens with ether. Form a flat spiral of two turns and approx. 12.5cm diameter from a length of about 75cm of platinum wire of 0.6mm diameter and place centrally in a shallow dish. Place six of the discs in a circle on the platinum wire and pour 150ml of hydrochloric acid (750g/l prepared by diluting 750ml of the concentrated acid (d 1.16) to 1 litre with water) into the dish. When the tin coating is dissolved (3-15 min according to its thickness) transfer the acid to a 1 litre volumetric flask, wash twice with 25ml of water, transferring the washings to the flask. Repeat the procedure with successive lots of six discs, combining the acid and washings in the same flask and finally diluting to the mark with water. 1 C /cm2 (10 mA/cm2 for 0 60 120 180 100 sec) removes 6.15 g/ TIME (T), s m2 of free tin or 4.06 g/Potential/time curve for m2 of tin in alloyed form coulometric measurement of coating thickness (this weight of alloyed tin on tinplate with corresponding rate of change corresponds to 5.06 g/m2 of potential/time curve. tin-iron compound). A current density may be chosen and retained to provide easy direct conversion of time interval to result, but it is always possible to prepare tables or graphs to aid the conversion
Transfer a 100 ml aliquot of the solution to a 750 ml conical flask, add 30 ml of hydrochloric acid (d. 1.16), dilute to 250 ml and heat to nearly boiling. Add 2 g of aluminium (99.5% , tin free, and in the form of heavy foil, coarse millings or drillings) in small amounts, and before complete dissolution of the last addition of aluminium, close the flask with a Contat-Göckel trap filled with a saturated solution of sodium hydrogen carbonate. Boil gently for 30 min to redissolve the precipitated tin metal, cool slightly and close the outer tube with a rubber cap. C ool in running water to room temperature (20°C ), remove the trap, add 5 ml of a 1% starch solution and 10 ml of a 10% potassium iodide solution and titrate with standard potassium iodate solution to a permanent blue colour.
The procedure differs slightly depending on whether electrolytic, differential, or hot dipped tinplate is to be determined. Thus for equally coated electrolytic tinplate, 12 specimens from four sheets are taken and the standard potassium iodate solution is 0.05 N* (1.7835 g/l; 1 ml ≡ 0.0029675 g Sn). For differentially coated tinplate, a similar 12 specimens are taken, but each surface coating is determined separately, the alternate side being masked with a suitable lacquer. The alternate face is subsequently determined, using the same (delacquered) specimens. The standard potassium iodate solution is 0.025 N* (0.8918 g/l; 1ml ≡ 0.00148375 g Sn).
The average coating mass is given by the expression, 3 2 5.935×10 VN Tin coating mass (g /m ) = A where: V = volume of potassium iodate in ml N = normality of the potassium iodate solution A = total specimen area in cm2 *The term “normality” rather than “molarity” is used with regard to the strength of the KI03 solution because this, together with the factor for tin (1 mI of 0.1N KI03 ≡ 0.005935 mg Sn) indicates to the analyst the reaction being used in this titration.
- THE BENDIX METHOD
(Ind. Eng. C hem. (Anal. Edn.) 1943, 15, 501).
This procedure may be used as a reasonably rapid test to check the validity of tests such as the X -ray fluorescence method, or where complex instrumentation is not available.
In this method tin from the test piece is anodically dissolved in dilute hydrochloric acid solution containing a measured excess of standard potassium iodate-potassium iodide solution. Excess iodate from the iodate-iodide solution is then back-titrated with sodium thiosulphate solution using a starch indicator.
Care is needed during the anodic dissolution process to determine the point at which the coating is fully removed. It is possible to halt the dissolution when only the free tin has been removed; however, the point at which the alloy layer is completely dissolved is not easily detected visually; since continuing the electrolysis further leads to dissolution of the steel base, erroneous values may result.
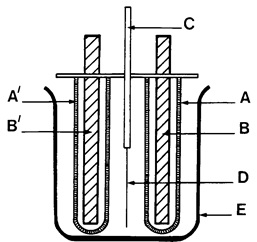
FIGURE 36
- A and A’ – Porous filters filled with 1:4 HCl
- B and B’ – cylindrical carbon cathodes
- C – Small electromagnetwhich holds sample at its edge without covering the tin surface
- D – Tinplate sample
- E – Containing beaker
DETINNING CELL
The detinning cell consists essentially of two carbon rod cathodes (e.g. 150 mm x 6 mm diameter), each enclosed in a porous pot (e.g. 120 mm x 20 mm diameter). These are mounted about 25 mm apart on a suitable framework, the test sample being suspended midway between the cathodes by a small glass enclosed magnet. A movable platform is arranged so that a 500 ml beaker of electrolyte can be brought up around the assembly so that the sample will be completely immersed (Figure 36).
- WEIGHT LOSS METHOD (CLARKE’S METHOD)
The weight loss method gives a very rough indication of the tin coating thickness. It is probably the simplest method to use when only occasional coating mass determinations are required. For modern, low coating weights the method is not suited. However, dissolving the tin coating in Clarke’s solution provides a useful practice to obtain the uncoated product. In this direct method, an addition of antimony to hydrochloric acid is used for the dual purpose of accelerating the dissolution of tin and of inhibiting dissolution of the steel base.
A sample of convenient size, say 50-100 cm2 is degreased, dried and weighed. It is then immersed in 100 ml of HC l containing 2 g Sb203 and allowed to remain in the stripping solution for about 1 minute after gas evolution has ceased. It is then washed, the black deposit of antimony removed by swabbing with cotton wool, dried and re-weighed. The weight loss represents the sum of the free tin and the alloy layer, so that a correction is needed for the amount of iron in the alloy layer, though for electrolytic tinplate this correction may usually be neglected. For hot dipped tinplate the correction is about 1.4 g/m2 and for electrolytic tinplate about 0.4 g/m2. If considered necessary the correction may be found from a determination of the alloy layer.
Assessment of tin oxide film
Tinplate surfaces are always to some extent covered by tin oxide which may contain one or both of stannous and stannic oxide together with their hydrated forms. Electrolytic tinplate, cathodically passivated, should have, immediately after treatment, only a very small amount of tin oxide on the surface and the measurement of this oxide may give some indication of the effectiveness of the treatment. A non uniform treatment or an over-long passage through the dichromate solution after the cathodic treatment section may produce abnormally high oxide. Although tin oxide may be measured on newly produced tinplate to help to check departures from correct procedure, the formation of added oxide during storage is most likely to cause troubles with appearance, lacquerability and solderability. Tin oxide measurements are therefore rather more valuable to users than to producers. Part of the object of passivation treatments is to prevent or restrict tin oxide growth and assessment of the passivation film itself, by chromium determination or resistance to heat discoloration, is a more useful means of assessing future behaviour than measurement of the initial tin oxide.
Positive identification of particular oxides is only possible by electron or X-ray diffraction and not always by these means, but it is more useful to have a measure of the quantity with some indication of the nature and the simpler means of controlled cathodic reduction can provide this information.
COULOMETRIC METHOD
- PRINCIPLE
Tin oxide is reduced by a controlled small cathodic current in a solution free from oxygen and inert to tin oxides. The progress of reduction of the oxide is followed by potential measurement and the charge passed for the complete reduction serves as a measure of the tin oxide on the surface.
- APPARATUS
The equipment is that described for general coulometric measurements with tinplate but provision must be made for removal of oxygen from the test cell and for the introduction into the cell of oxygen-free solution. With the type of cell described earlier, for coulometric measurements, the closure to the top of the cell is fitted with an exit and entry tube, through which nitrogen passes after bubbling through the test solution contained in a suitable flask.
- SOLUTION
0.001N hydrobromic acid, freed from oxygen by, for example, boiling and cooling whilst scrubbed with nitrogen. This solution is widely used for routine measurements and gives reliable results but other solutions may be used for research purposes, for example, a sodium phosphate solution buffered to near neutrality.
- PROCEDURE
When the apparatus is first set up, it is useful to carry out a dummy run with an oxidised tin surface to ensure that the current and potential range are correctly set. The directions given are those applying to the cell previously described, but the necessary modifications for any other form of cell should be clear. A convenient current density is 25 µA/cm2 and the potential range to be recorded will usually be 400-1000 mV negative to a calomel electrode.
Rinse the test piece with a neutral solvent and place it in the dry test cell. C lose the cell and pass through it a stream of nitrogen which has already bubbled through the test solution contained in a flask joined to the cell by a flexible tube. After two minutes, invert the flask containing the solution so that a quantity sufficient to cover the test piece and electrodes is forced into the test cell. The delivery tube should remain above the surface of the solution and should be partly withdrawn if too much is delivered. Switch on the recorder and then the current with the test piece as cathode. Record the potential until the reduction is complete. The reduction may take place at more than one potential and it is necessary to ensure that the potential corresponding to bare tin is reached.
- RESULTS
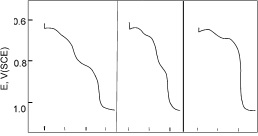
FIGURE 36
The curve showing the relation of potential to time frequently has two or sometimes three potential steps. The first two steps, occurring at -600 to -700 mV relative to saturated calomel, may refer to the reduction of
either stannous or stannic oxides. The 0 2 4 6 0 2 4 0 2 4 third step which rarely m in appears for electrolytic tinplate, but is often Forms of potential/time curve obtained for seen for hot-dipped reduction of tin oxide tinplate, relates to stannic oxide and may be associated with the oxidation of particular crystal faces. In view of the uncertainty of the oxide composition, it is common practice to record the oxide as a reduction value in mC /cm2 corresponding to the time interval between the start of current and the inflection in the potential/time curve immediately before the bare tin potential is reached. Examples of reduction curves are shown in Figure 37. The reduction values obtained for new electrolytic tinplate are usually less than 1 mC /cm2 and the possibility of some trouble arising from tin oxide may be expected when the value exceeds about 3 mC /cm2.
- ASSESSMENT OF CHROMIUM IN PASSIVATION FILMS
The passivation treatments applied to tinplate deposit on its surface a thin layer which contains chromium in at least two forms. Part of the chromium can be removed by extraction with hot alkaline solutions and the other part can be removed by anodic oxidation to the soluble hexavalent form or by extraction with mineral acid. It is likely that the fraction soluble in acids or by anodic oxidation is metallic chromium and that the remainder is a mixture of more or less hydrated oxides. Although doubts have been expressed about the nature of the two components, it is convenient to refer to one as chromium metal (C r°) and the other as chromium oxides (C rIIl). The resistance of the tinplate to sulphur staining benefits more from the presence of C r° than from the presence of C rIIl and there is therefore some interest in measuring the two components separately. However, the tinplate produced on one line with the same passivation conditions maintains a nearly constant ratio of the amounts of the two components and this same relationship may hold good for a number of other lines also operating a similar cathodic passivation process.
It is therefore possible to obtain a good approximation to the total amount of chromium on the surface by measuring only the component which may be oxidised anodically and this measurement may be made rapidly by coulometry. The coulometric method is suitable for most routine checks of production or of suitability for particular purposes but it will not yield information about the separate components. Other tests for determining the chromium on tinplate are by direct dissolution in sulphuric acid and determining the chromium contents by means of Atomic Absorption Spectrometry or by the chemical diphenylcarbazide method.
COULOMETRIC METHOD
- PRINCIPLE
One of the components of the passivation film and a small fraction of another is oxidised by anodic applied current to soluble hexavalent chromium. Because other oxidation processes are taking place, the theoretical yield from the charge passed on oxidation from C rO to C rVI is not obtained but a calibration made against chemical analysis holds good for a wide range of tinplates.
- APPARATUS
The general purpose coulometric apparatus may be used. Special requirements are that any reference electrode must not leak chloride appreciably into the test solution, although a saturated calomel electrode with one of the better wick-sealed capillaries on its sheath is satisfactory, and that the potential measurer shall be capable of covering a range up to + 2000 mV.
- SOLUTION
A phosphate buffer of pH 7.4 is suitable and may be prepared by mixing 200 ml of a sodium dihydrogen phosphate solution, 8 g/l, with 800 ml disodium hydrogen phosphate solution, 9.5 g/l. Other solutions may be used but the pH should be between 6 and 8.5 and the calibration should be made for the solution that is to be used.
- PROCEDURE
Set up the apparatus to give a constant anodic current of 25 µA/cm2 on the selected test area and to make possible the recording of potential change from -200 mV to + 1500 mV.
Place the specimen in position in the electrolysis cell add the test solution and put the electrodes in place with solution open to air. (It is not usually necessary to clean test material but sometimes especially heavy oil films, some form of contamination or lacquer may have to be removed and this should be done only by the use of solvents, since electrolytic cleaning or strong alkalis may remove some chromium.) Switch on the current and continue until the potential against saturated calomel electrode is in the region + 1300 to 1500 mV with a straight line relating time to potential over the final 200 mV at least
- RESULT - TIME
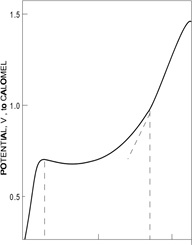
T he typical potential/time curve obtained (as Figure 38) shows a sharp rise in potential with a sudden break, often with a cusp, to a stage of nonexistent or much slower change of potential as the chromium is oxidised, usually in the region of + 700 mV. As oxidation proceeds the potential begins again to increase more quickly and a region of linear potential/time relationship is reached before ultimately
0 180 360 there is a further step of very slow TIME .s 0 potential increase when oxygen Figure 38 evolution starts. The time taken by the Potential/time curve for the coulometric oxidation of the chromium is the oxidation of chromium in the passivation layer of tinplate interval between the first sharp break and the final change to a linear relationship. For the solution and conditions described, a typical value equivalence would be that 1 mC /cm2, i.e. 40 sec. at 25 µA/cm2 indicates 0.1µA/cm2 of chromium on the surface. It must, however, again be stated that this is an approximate measurement depending for its accuracy on calibration against a chemical method.
- DIPHENYLCARBAZIDE METHOD
The chromium on tinplate may also be determined by a chemical method. The diphenylcarbazide method is described in detail in the American Specification ASTM A623:98. For completeness, brief details of the method are given below.
A sample disc having a diameter of 5.75 cm is used. This provides a surface area on each face of 26 cm2. (Total surface 52 cm2.)
Place the sample in a beaker, with 25 ml of a mixed sodium hydroxide, trisodium phosphate solution (1 N NaOH, 5% Na3PO 4,) and boil for about 1½ min.
Transfer the alkaline solution to a fresh vessel. To the original beaker and sample, add 25 ml H 2SO 4, (25% solution by vol.) and boil again. C ombine the two solutions again, bring to boiling point and add 1-2 drops KMn04 solution to produce a pink colour. Boil to oxidise the chrome (usually 3-4 min). Add 5 drops HC l (SG 1.19) and continue to boil until pink colour disappears.
Transfer to a 100 ml volumetric flask, cool to approximately 21°C . Add 3 ml diphenylcarbazide reagent, make up to the mark with water.
Determine the optical density at 540 nm. The ASTM recommends carrying out the tests in conjunction with reagent blanks and a standard using all solutions employed. The spectrophotometer is calibrated against standard K 2Cr2O 7, solutions.
ASTM A623:98 also includes formulae for the calculation of the amount of chromium on tinplate in terms of micrograms chromium per square foot of surface:
Calibration of spectrophotometer:
Calculate a constant, K for the instrument as follows:
K = (µg C r) / (O.D.1 – O.D.2)
Where: O.D.1 = optical density for the standard, and O.D.2 = optical density for the blank.Calculation of chromium on tinplate:
Report chromium on tinplate as micrograms of chromium per square foot of surface area, as follows:
Cr, µg/ft2 = [144 K (O.D.1 – O.D.2)) /A
Where: K = constant for spectrophotometer and cell used to determine optical density
O.D.1 = optical density of sample
O.D.2 = optical density of reagent blank, and A = area of sample used in ft2.
- SPECIAL PROPERTY TESTS
This group of tests has been designed to assess characteristics that influence the shelf lives of citrus fruit packs in plain cans. Special property test values are not normally specified in national or international standards, although detailed procedures are described in ASTM A623. These properties are only specified and tested in very specific purposes. In this overview only a rough description is given. More specific details can be found in the standard given above.
- THE ALLOY TIN COUPLE (ATC ) TEST
The ATC test is designed to measure certain characteristics of electrolytic tinplate affecting internal can corrosion resistance. The test is applicable to tinplate having a nominal coating mass 5.6/2.8 g/m2 or heavier. It is not applicable to 2.8/2.8 g/m2 or lighter coated tinplate.
The ATC test is an electrochemical procedure which involves measuring the current flowing between a pure tin electrode and an FeSn2 electrode, prepared by removing the free tin from a specimen of tinplate so as to expose the alloy layer. The current measurement is made after 20 hours exposure of the electrodes in an electrolyte comprising de-aerated grapefruit juice.
A serious drawback to the test is its dependence on a natural medium. However, synthetic electrolytes not only give numerically different results, but also may put a range of tinplates into a different order of merit.
- THE AERATED MEDIUM POLARISATION (AMP) TEST
One disadvantage of the ATC test is the considerable time (20 hr) taken to obtain results. The AMP test has been developed which can give a rough guide within a matter of minutes.
In this test, a specimen prepared as for the ATC test is cathodically polarised at high current density, the high polarisation current serving to remove oxygen from the grapefruit juice medium near the surface of the electrode.
For a given current density, the polarisation (change in potential after current is switched on) depends on the proportion of steel exposed, as steel is less strongly polarised than iron-tin alloy.
The AMP value may then be converted to an “ATC value” using calibration curves prepared by running the two tests with the same material but it should be emphasised that “ATC values” obtained in this way have no validity on their own account, being merely rapid estimates of what the true ATC value may turn out to be.
- IRON SOLUTION VALUE (ISV) TEST
Like the ATC (and AMP) tests, the ISV test is only applicable to the heavier coated tinplate grades. The ISV test involves the colorimetric determination of the iron dissolved when a controlled area of tinplate is exposed for two hours at 27°C to 50 ml of a solution that is a mixture of dilute sulphuric acid, hydrogen peroxide and ammonium thiocyanate. The ISV is defined as the number of micrograms of iron dissolved.
- THE PICKLE LAG TEST
The pickle lag test (also referred to as Rate of Pickling Test or Induction Period Test), is a test performed on the steel base of tinplate (i.e. on detinned material). Nonetheless as for the other special property tests, the test results should not be considered to be significant for the lower coating mass tinplates.
- TIN CRYSTAL SIZE (TCS) TEST
For some purposes it may be desirable to measure the grain size of the free tin surface (TC S). This is readily done by lightly etching the surface with a suitable etchant and comparing the grain size against standard grain size charts (ASTM), a useful predictor of tinplate performance. A suitable etchant contains in one litre, 84 ml concentrated hydrochloric acid, 100 g hydrated iron (III) chloride and 0.5-1.0 g sodium sulphide or sodium bisulphite. Although the solution is reusable, it should be replaced when etching of a correctly prepared specimen takes longer than 30 seconds or when the solution turns green.
Samples of about 25 cm2 area are taken, identification marks being put on the face opposite to that to be tested. Samples are cleaned cathodically in 0.5-1% sodium carbonate solution for 15-30 seconds at room temperature, rinsed and dried. The cleaned samples are then dipped into the etching solution until the grain pattern can just be seen (usually 5-15 seconds), withdrawn, rinsed thoroughly in water and dried. The tin crystal size, as viewed with the unaided eye is compared directly with the ASTM macro grain size number standards (test method ASTM E112). The result is usually expressed as a grade number on the ASTM chart, and for tinplate a typical number is in the range 6 to 12.
- POROSITY TESTS
In general, the porosity of a plated coating is inversely related to its thickness. However, for comparison purposes it is sometimes desirable to assign a numerical value to, or to assess visually, the porosity of a given sample of tinplate. There are many suitable tests, among them the following.
- THIOCYANATE TEST
The test sample is immersed in an acid solution containing ammonium thiocyanate designed to be inert to tin but aggressive to steel exposed at coating pores. The amount of iron entering solution is estimated colorimetrically as iron (III) thiocyanate.
Scratch-free tinplate samples, say 100 mm by 50 mm in area, are selected to be representative of the undamaged surface. The samples are first cleaned by a cathodic-anodic-cathodic treatment in 1% sodium carbonate solution at room temperature. This treatment removes deposits introduced into pores by the passivation treatment. After rinsing and drying, cut edges of the sample should be coated with wax or lacquer. The sample supported by non-metallic material, should then be immersed in the test solution (250 ml) left undisturbed for 15 minutes then moved briskly in the solution and removed. The test solution consists of 100 ml thiocyanate solution (50 g of ammonium thiocyanate in 1 litre of water), 100 ml acetic acid solution (25 g acetic acid per litre of water) and 50 ml hydrogen peroxide solution (3 g hydrogen peroxide per litre of water). After removal of the sample, add 5 ml of dilute sulphuric acid (400 ml concentrated sulphuric acid in 1 litre of water) and compare the intensity of the red colour with that produced by standard solutions. The results of the thiocyanate test are normally expressed as mg Fe/dm2 of surface.
- SULPHUR DIOXIDE TEST
The sample is exposed for a fixed period to an atmosphere containing a small amount of sulphur dioxide. Under these conditions, rust spots form at pores, whereas areas of continuous tin coating are unaffected.
The test sample is first cleaned cathodically-anodically-cathodically in 1% sodium carbonate solution, as for the thiocyanate test, and then well rinsed (in water, followed by acetone or ethanol) and dried.
Test samples are suspended, by means of glass hooks hanging from a plastic frame, within a vessel fitted with an airtight closure. The vessel contains a volume of sodium thiosulphate solution (10 g sodium thiosulphate per litre water) equal to 1/20th of the vessel’s capacity, to which has been added 0.1 N sulphuric acid (4.9 g/l H 2SO 4) in the ratio 1 part acid for every 10 parts thiosulphate solution. The ambient temperature is maintained at 23± 3°C . The samples are suspended in such a way that no part of their surface will be more than 300 mm or less than 100 mm from the surface of the solution, making sure that each specimen is separated from its neighbours or the wall of the vessel by at least 20 mm. After adding the sulphuric acid, the specimens are suspended in position within 5 minutes, the vessel is then closed and left undisturbed for a suitable period. Usually 16-24 hours is sufficient to allow rust to develop at all pores, if longer periods are employed, spread of rust may obscure single pore sites. At the end of the test period, the test samples are removed and inspected.
They may be compared with previously prepared reference samples; alternatively a pore count may be made. Because pore densities may be of the order of 1000/cm2, it may be impracticable to count each pore separately; the surface may be covered by a grid marked in 0.5 cm squares, and the pores counted at a magnification of x5 on a number of squares distributed at random over the test area.
The sulphur dioxide test can also be used as a direct measure of the corrosion resistance of passivated tinplate. In this case, the test pieces are not cleaned before exposure, and the exposed samples are compared with reference standards. Surface heterogeneity revealed in this way can be of value in indicating defects in the steel substrate or variations in the passivation or oil films.
Sulphide stain resistance
One of the functions of surface chromium metal in passivation films is to protect against the formation of unsightly tin sulphide deposits that may form during the heat processing of canned meats or other sulphur-containing foods. Surface chromium content is one useful guide to the likely resistance to sulphide staining (about 1 µg chromium/cm2 being needed for good resistance); another direct measure of sulphide stain resistance is the cysteine staining test. Cysteine is a sulphur-containing amino acid, present in some natural products, and the test involves exposure of samples to neutralised cysteine solution at 110°C for 30 minutes under pressure. The test solution consists of cysteine hydrochloride solution (3g per litre in distilled water of low oxygen content) neutralised to pH7 by addition of 0.2 M trisodium phosphate. The test is carried out in fruit-preserving jars within a pressure vessel. The test-pieces are circular 50 mm diameter discs or squares of side 40 mm with central holes to accommodate 2-3 mm diameter glass rods. Spools of samples are prepared by threading the discs on glass rods that are expanded at one end and slipping 15 mm long lengths of tubing after each sample to act as spacers. Blank specimens are fitted to each end of each rod.
The jars and pressure vessel are heated to 100°C , the cysteine solution, just neutralised, being brought to the boil in a separate beaker. A spool of samples is placed in a jar which is then flushed through with nitrogen. The hot cysteine solution is poured into the jar which is quickly transferred to the pressure vessel full of boiling water. When all the jars for the test are in place, the pressure vessel is closed and, after venting of air, its temperature is raised to 110°C , this temperature being maintained for 30 minutes. After releasing the pressure, the vessel is opened, the jars removed and allowed to cool. When cool enough, test samples are removed, rinsed with hot water, dried and inspected. The sample may either be compared with a set of standards or the sulphide estimated coulometrically by reduction to tin metal.
If a quantitative result is required, the sulphide on the surface of each specimen is measured. This may be done chemically, but a coulometric estimation is convenient. For coulometric measurement, the apparatus described earlier for general coulometric measurements may be used. The area which can be tested is limited by the form of test piece and usually 2 or 3 areas of 10 mm diameter are examined on each specimen. The electrolyte is deoxygenated 1 N sodium carbonate solution and the current density is 250 µA/cm2.
- RESULTS
For simple inspection procedures, the test samples may be compared with set standards. For coulometric measurements, the sulphide reduction curve is usually two-stepped; the first, least negative step often appearing as a sharp cusp at -1250 to -1300 mV, is the reduction of oxide and the second step, in the region -1350 to -1600 mV relative to the saturated calomel electrode, must be taken. The step is usually spread over a potential range but the inflections of the time/potential curve which mark its start and end are sufficiently well defined. The result may be expressed as mC /cm2. The sample with a value above 5 usually has a visible stain and a value above 10, a severe stain.
- OIL FILM DETERMINATION
The oil film customarily present on electrolytic tinplate is dioctyl sebacate or acetyl tributyl citrate.
The simplest test, which merely indicates the presence or absence of oil, is the “water-break” test, in which tinplate sheet is momentarily dipped in water, and then examined for areas where the water remains. There are, however, a number of quantitative tests which may be applied. The results of these are sensitive to the treatment received by the sheet in question, for example a single sheet withdrawn from stack and exposed to air for only a short while may give a different result from others left in the stack. To reduce these problems, a convenient practice in sampling for oil film determination is to take a sample of three consecutive sheets, using the outer two as cover sheets and making determinations on the inner sheet only. The cover sheets should be retained as far as possible during the cutting operations and used for carrying the sample sheet.
- ELLIPSOMETER
The most common method employed for oil film determination used by tinplate producers and many major consumers makes use of an instrument know as the ellipsometer.
This is an adaptation of an optical method used for examining thin films on metal. When polarised light is reflected from a surface, the components parallel and perpendicular to the plane of incidence undergo relative changes of phase and amplitude. If the surface is covered by a transparent film, the extent of each of these changes is affected by the thickness and refractive index of the film. If the incident light is plane polarised, the reflected light is elliptically polarised, to a degree which can be measured and hence the film thickness can be determined. This measurement is made in the ellipsometer.
To determine oil film thickness by the technique, the reflections are obtained from the same spot on a piece of tinplate both before and after the oil film has been removed (by a solvent). The results are compared and used to determine the oil film thickness. The instrument is calibrated using one of the basic methods.
- THE SOXHLET METHOD
This is the most basic method of measuring the oil film on tinplate. Formerly used as a routine control method, it has now largely been superseded by more rapid methods. It is however, described in ASTM A623, where full details of the procedure may be found.
The principle of the method is that the oil on a known area of tinplate is removed with a boiling solvent (ASTM specifies chloroform or an equivalent solvent). The solvent containing the oil is then evaporated to dryness and the residue (the extracted oil) is weighed. The determination is carried out in parallel with a blank.
- THE HYDROPHIL BALANCE
This method, or a modification of it, may be used to determine oil film mass. It is sensitive to “ageing” of the oil.
The Hydrophil balance method involves the spreading of the oil as a monomolecular film on a clean water surface, the area covered giving a measure of the mass of the oil. The equipment is a slightly modified Langmuir trough obtainable from certain suppliers of specialised test equipment. It consists essentially of a rectangular shallow tray, filled with water, a light strip floating on the water so as to extend across almost the whole width of the tray, whose movements, indicated by a light vane, can be controlled by a torsion wire with a regulating torque head. A sliding barrier rests on the tray’s edges, and there is a well about 90 mm deep and 10 mm wide in the bottom of the tray. After filling the well-cleaned tray with distilled water, the water surface is swept by several movements of a slide to remove surface contamination. The moving barrier is placed at the end of the tray far from the float and a tinplate sample of known area is vertically passed through the water surface into the well and then withdrawn. This procedure is repeated 5 or 6 times for the same sample, allowing 8-10 seconds for each cycle. The effect of this operation is to transfer a high proportion of the oil from the tinplate to the water surface. The torque head controlling the floating strip is turned through 3°, so deflecting the indicating vane attached to the float. The sliding barrier is then moved towards the float until the indicating vane returns to the zero position, the position of the sliding barrier then being noted. The procedure should be repeated for at least 5 samples from the tinplate sheet under test. The hydrophil balance is calibrated by carrying out measurements on known quantities of the same oil. A convenient calibration solution contains 0.5 g/litre of oil in benzene; this is applied to the water surface with a micro-syringe able to dispose multiples of 0.01 ml. Because the areas occupied by monomolecular oil films are directly proportional to the masses of oil involved, the calibration result can be expressed as mass of oil per unit length of tray, and the measured length of the unknown oil film can be converted directly to mass of oil on the sample, the result being expressed in grams per square metre.
A variant of the Hydrophil balance method is to extract the oil from a known area of tinplate, using a suitable solvent, and to micro-pipette an aliquot on to the water surface, as described in the calibration procedure. This minimises difficulties due to the changes in characteristics of the oil due to ageing.
- SOLDERABILITY TESTING
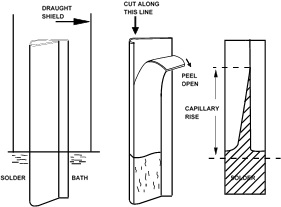
An important attribute of tinplate is its ability to be soldered with ease and rapidity. Although general solderability testing methods involving wetting time or area of spread measurements are also applicable to tinplate, there is a simple capillary rise tinplate solderability test that is commonly used.
In this capillary rise test, a strip of tinplate, about 75 mm long by 25 mm wide is folded lengthwise to form a tube of pear-shaped cross-section such that the gap between the opposing surfaces tapers from 0 to about 5 mm. The gap is fluxed and one end of the specimen is immersed vertically to a depth of about 30 mm in a small bath of molten solder (Figure). Figure Simple test for assessing solderability by observation of rise of molten solder within a fold of tinplate
A draught shield is used to minimise temperature fluctuations. After a brief period, which may be varied to obtain a relation of the result to time, the folded test-piece is withdrawn and allowed to cool. The fold is slit open and the height to which the solder has risen in the capillary is measured. This test gives a relative assessment of solderability, it does not provide absolute values.
The wetting balance has also been used in solderability testing. This measures the wetting forces which occur when a specimen is immersed in a solder bath and records them as a function of time. When the specimen is first immersed, a buoyancy upthrust occurs giving a positive force that changes to a negative force as wetting takes place. Eventually the solder rises up the specimen to reach an equilibrium position. Measurements frequently taken are the elapsed time from the point of immersion for the wetting force to pass through the upthrust stage and to return to zero force. In the case of differentially coated tinplate, a more complicated procedure is needed and interpretation of results is more difficult.
Joint strength is an important parameter and a number of tests may be used to assess this. Normally such laboratory tests involve making a controlled lap joint and testing it by tearing in a tensile test.
In one such test two strips of tinplate, about 75 mm X 25 mm are used. A strip of aluminium foil is wrapped around one of the tinplate strips so as to leave the centre portion bare. This area is fluxed and the other tinplate strip placed against it; the assembly is clamped together, in an unsolderable jig, to leave a controlled gap. The whole is immersed in molten solder, withdrawn and allowed to cool. The aluminium foil is removed and two unsoldered ends parted, to give a T-shaped specimen. The free ends are clamped in a tensile machine, and the force required to peel open the joint is measured. The test values depend on a number of factors, including coating mass, tinplate thickness and temper grade. It can however, provide a useful guide to the soldering properties of a given specification of tinplate.
In practice, it is rare to obtain poor test results with tinplate. Where poor joint strength is found, poor steel preparation or discontinuous or otherwise abnormal iron-tin alloy layer may be responsible.
Next:
Latest news
-
Affordable Used Car Engines Prices Quality Used Car Engines for Sale Reliable Used Engines
NewsJul.08,2025
-
Can You Use Dish Soap on Cars? Discover Safe Car Cleaning Alternatives
NewsJul.08,2025
-
Top Car and Driver EV SUV Picks Best Electric SUVs 2023, Ratings & Reviews
NewsJul.07,2025
-
How to Buy Used Cars Cheap Best Places & Top Deals for Affordable Vehicles
NewsJul.07,2025
-
Best Danbury Used Cars for Sale Reliable Used Cars Danbury CT Dealer Ingersoll Auto Specials
NewsJul.06,2025
-
Quality Used Car Parts in Asheville Affordable Asheville NC Auto Parts Reliable Asheville Used Car Dealerships
NewsJul.06,2025
